The human heart is a very interesting and well-synchronized electrical device. Every heart beat begins with an electrical signal, which originates at the sinoatrial node, and then makes its way first across the atria and then across the ventricles. The propagation of this signal across the myocardium generates the well-known ECG signal.
The arrival of the electrical potential signal results in the contraction of the cardiac muscle, producing the mechanical pumping action of the heart. It is therefore imperative that the electrical wave propagation across the myocardium be well-synchronized, for optimal blood supply to the body and disruption of this pattern results in pathological conditions like Ventricular Tachycardia. Cardiac Electrophysiology is the study of the electrical conduction system of the heart, its pathologies and corresponding therapies.
For improved patient-specific diagnosis and therapy planning, computational models of the cardiac electrical potential propagation have been proposed. These models are commonly developed using either the Finite Element Method (FEM) which require significant computational resources, or the Finite Difference Method (FDM) which tend to be much faster but have difficulty with the complex geometric structure and anisotropic material properties of the cardiac tissue.
Using a new computational method called the Lattice-Boltzmann Method, our team developed an extremely fast model for cardiac electrophysiology which is also able to deal with the complex shape and anisotropic conductivity of the heart. This method has the advantage of being inherently parallelizable, and can easily take advantage of modern hardware architectures like Graphical Processing Units. While it was originally developed for fluid flows, our team adapted it to the anisotropic equations of Cardiac Electrophysiology and augmented it with a level-set based method for dealing with the complex geometry of the heart.
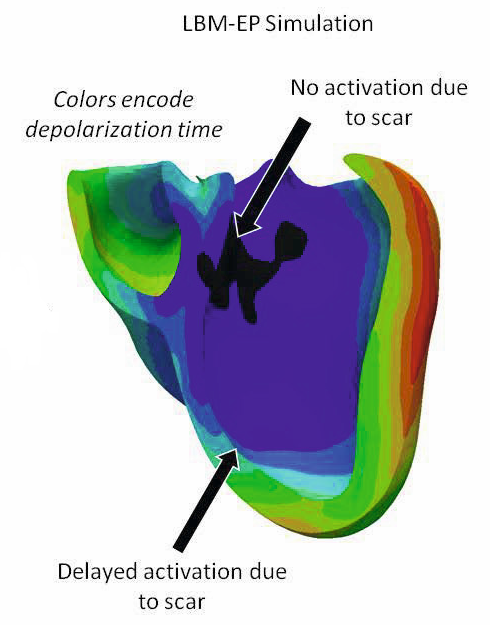
Using this new formulation, we increased the performance of electrophysiology models by a factor of 80, obtaining nearly real-time evaluation of patient-specific models (initial results are presented here). This has enabled the development of a comprehensive prototype for the patient-specific study of cardiac electrophysiology.
Acknowledgements
This work was jointly done in collaboration with Tommaso Mansi, Bogdan Georgescu and Tiziano Passerini.