Coronary Artery Disease (CAD) is characterized by the narrowing of arteries which supply blood to the heart due to a buildup of plaque. It is one of the leading cause of death, globally. Under conditions of rest, the heart is able to regulate sufficient blood flow to itself despite the presence of the obstructions. However, under conditions of stress, the oxygen demand of the heart tissue increases and the heart may not be able to maintain sufficient blood flow to the heart.
The image below (source: Wikipedia, donated by Blausen Medical to Wikipedia) shows an obstruction in one of the coronary arteries leading to cardiac muscle damage as the heart is not able to supply enough oxygen to the regions downstream to the obstruction.
If the obstruction in the artery is deemed as being significant, the vessel is opened up (revascularized) through stenting. Multiple studies have shown that, compared to typical anatomical quantification (for instance, percentage reduction in vessel diameter or area), a functional measurement called Fractional Flow Reserve (FFR) provides much better patient outcomes as well as lower costs. FFR is computed invasively using a pressure-sensing catheter, by taking the ratio of average pressure after the obstruction to the average pressure upstream to the obstruction.
We developed a fast, completely non-invasive model for computing FFR from Coronary CT Angiography (CCTA) images of a patient. CCTA is a modality with excellent ability to identify patients without significant CAD, but lacks specificity in identifying patients who would benefit from receiving a stent. Mutiple research groups 1 have shown that the specificity of CCTA imaging can be significantly improved by combining the coronary anatomy from CCTA images with mathematical models describing the flow of blood along with suitable boundary conditions. However, these blood flow models can be computationally quite expensive, requiring anywhere between a few minutes to a few hours depending on the complexity of physics in the model.
As an alternative approach, we investigated the use of Machine Learning for predicting the pressure losses due to coronary obstructions. To train the model, we created a large database of synthetically generated coronary artery anatomical trees, with published statistical distributions for the anatomical features. On each of these anatomical models, a blood flow model was used to compute pressures everywhere. The Machine Learning model was then trained to learn the mapping between the anatomical features describing the geometry of the vessel tree at each point, to the computed pressure at that point.
The model was tested by comparing its predictions to those obtained using a blood flow model on a sample of real patient anatomies. It is worth noting that the machine learning model was only exposed to synthetically generated trees until this point, and had never encountered patient data before. We showed an excellent agreement between the ML and blood flow models (>99.9% correlation). Further, the average computational run time of the ML model was only 2.5 seconds per patient, compared to nearly 200 seconds for the blood flow model.
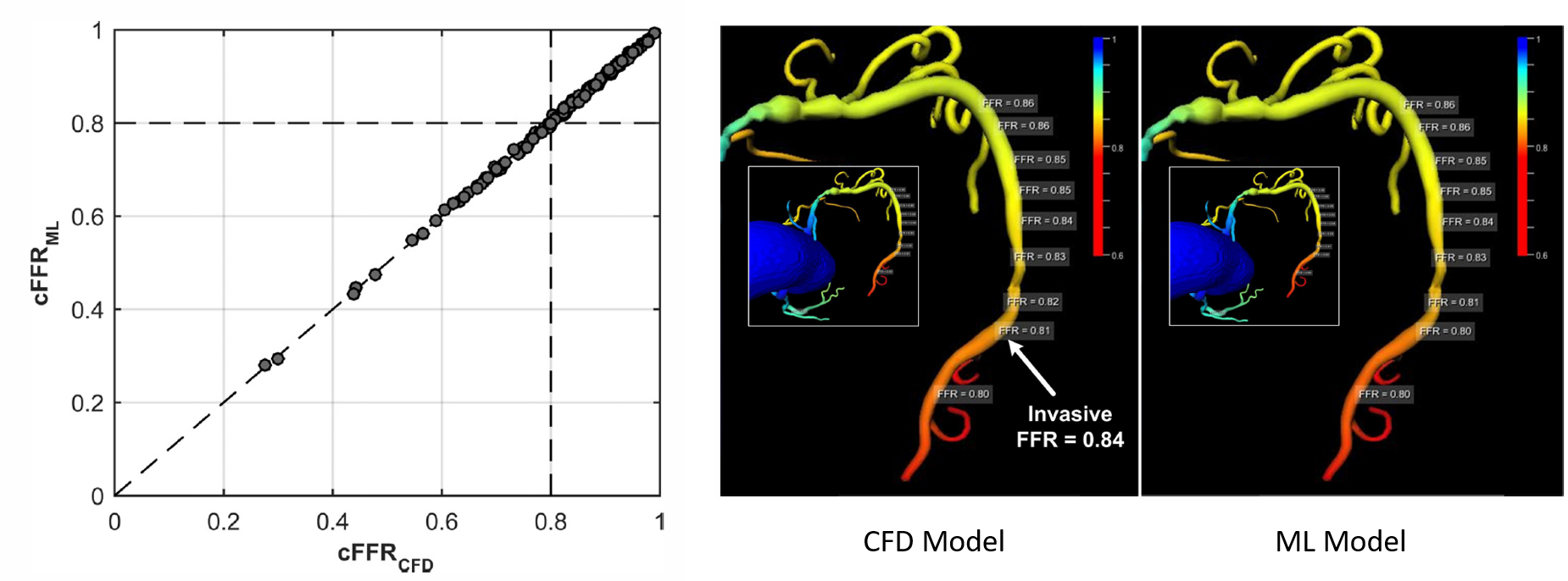
In the figure above, the left panel shows a comparison between the FFR predicted by the CFD and ML models for a set of 87 patients, and the center and right panels show the spatial variation of FFR predicted by the models for one representative patient.
Acknowledgements
This work was jointly done in collaboration with Lucian Mihai Itu, Tiziano Passerini, Bogdan Georgescu, Chris Schwemmer, Max Schoebinger, Thomas Flohr, Puneet Sharma and Dorin Comaniciu.
Related publication
Patents
- Synthetic data-driven hemodynamic determination in medical imaging
- Computation of hemodynamic quantities from angiographic data
- For instance, Coenen et al, Radiology (2015), Min et al, JAMA (2012), Morris et al, JACC: Cariovascular Interventions (2013), Norgaard et al, JACC (2014), Papafaklis et al, EuroIntervention (2014), Renker et al, Am J Cardiol (2014), Wang et al, Eur J Radiol (2015). [return]